| Cat. No. | Species | Product Description | Structure | Purity | Feature |
|---|---|---|---|---|---|
| VE1-H5253 | Human | Human VEGF R1 Protein, Fc Tag |  |
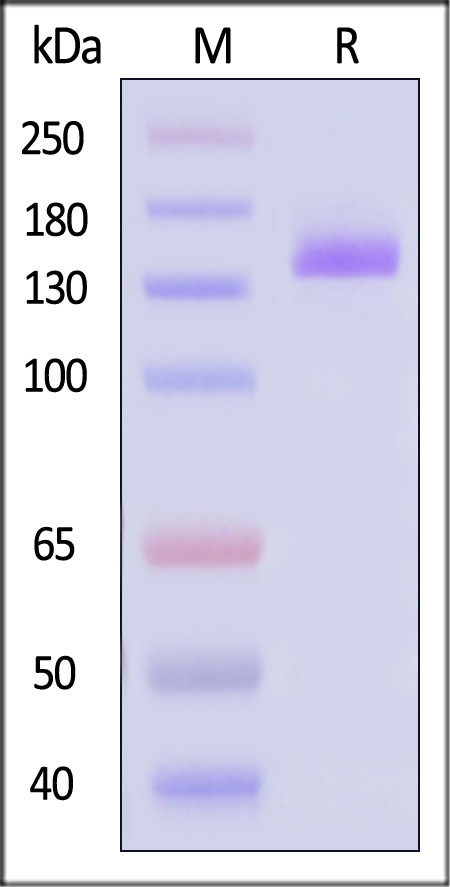
|
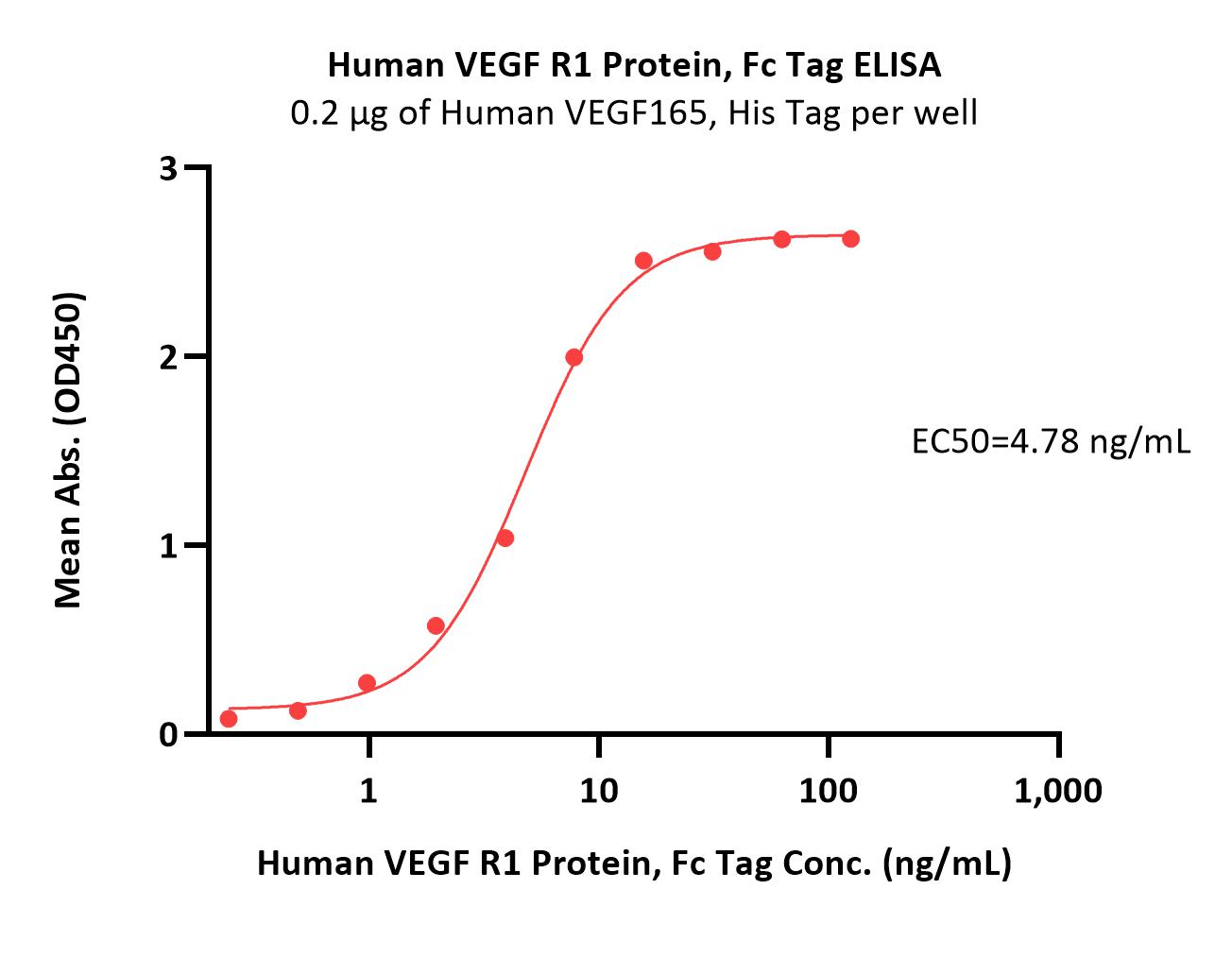
|
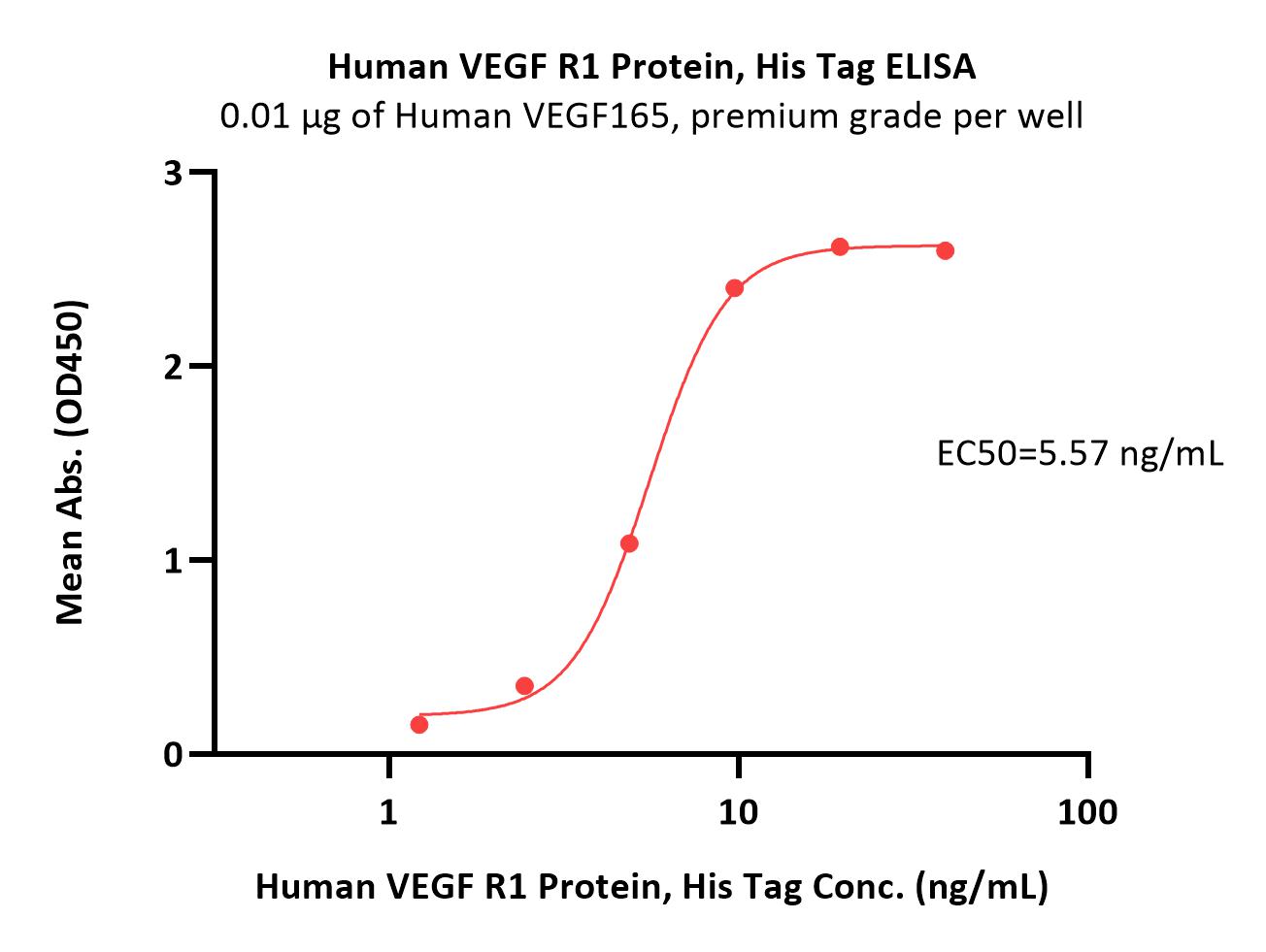
| VE1-H52H9 | Human | Human VEGF R1 / Flt-1 Protein, His Tag (MALS verified) |  |
|
|
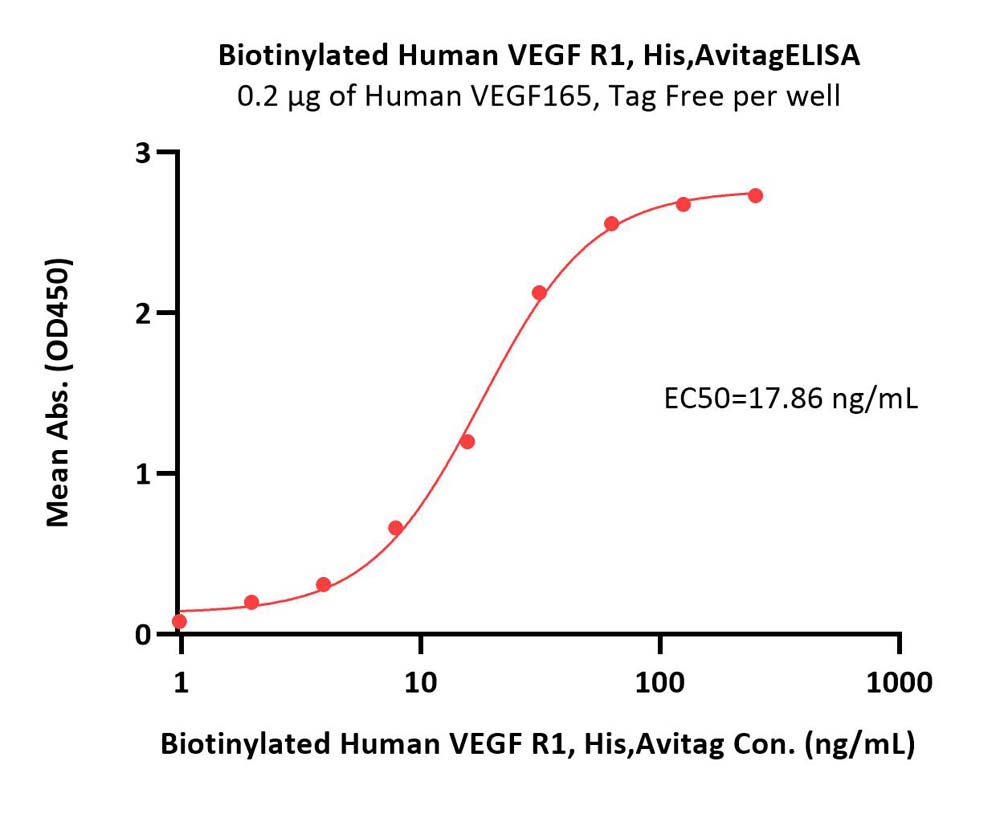
| VE1-H82E3 | Human | Biotinylated Human VEGF R1 / Flt-1 Protein, His,Avitag™ |  |

|
|
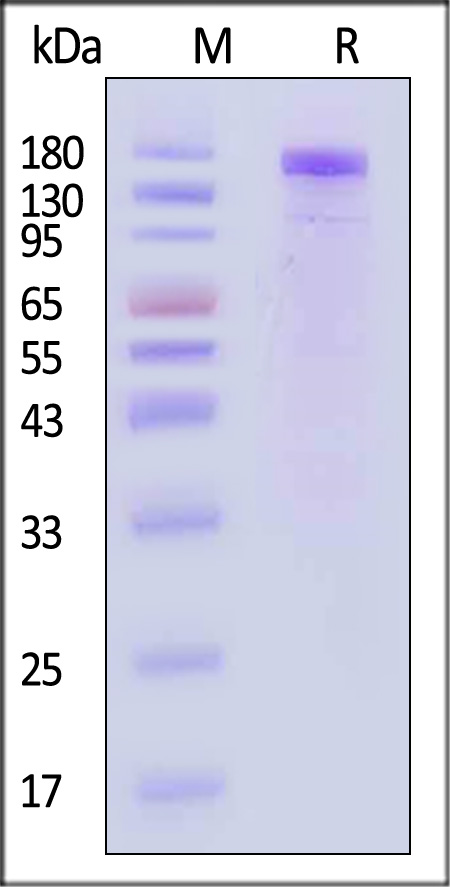
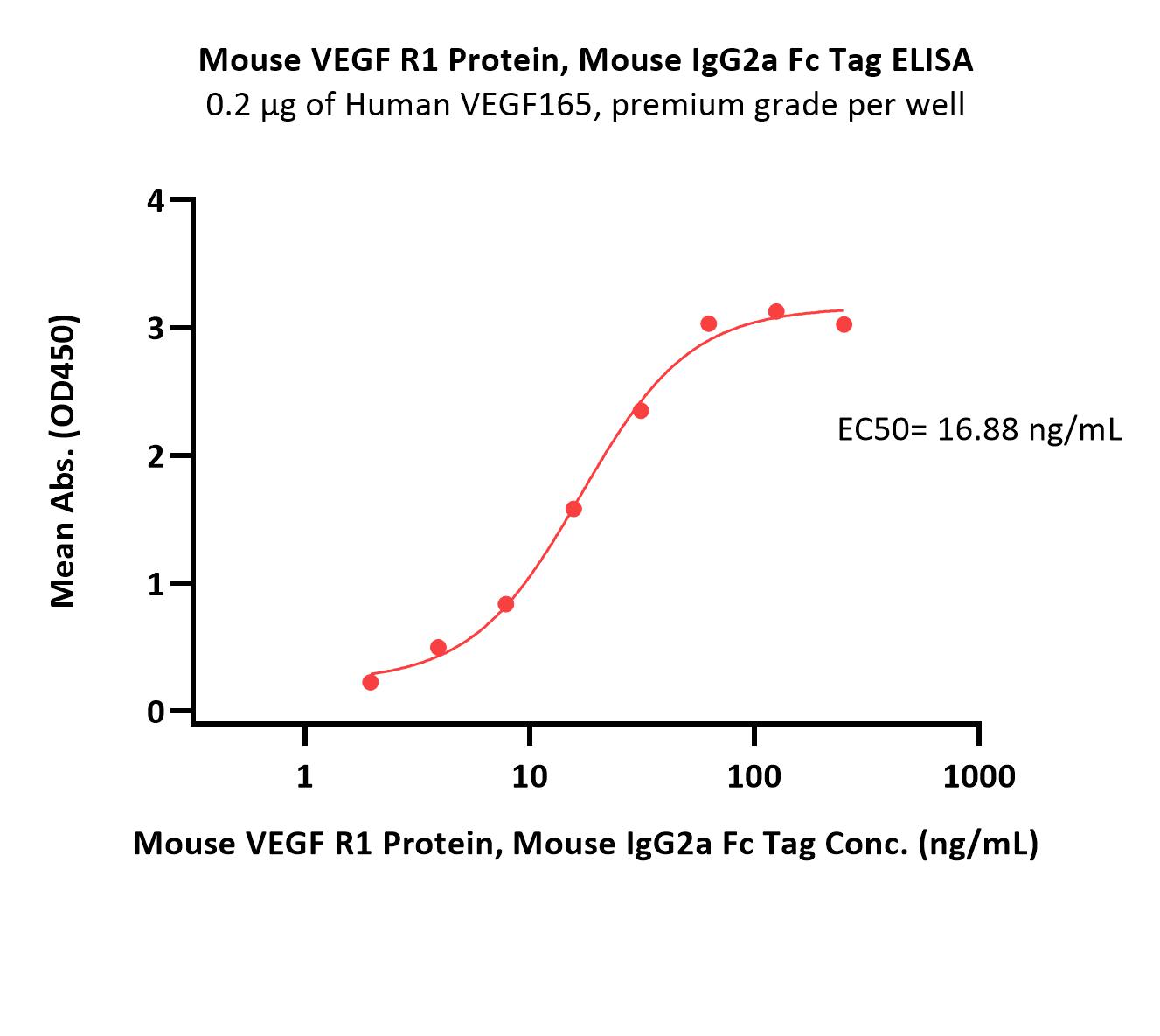
| VE1-M5256 | Mouse | Mouse VEGF R1 / Flt-1 Protein, Mouse IgG2a Fc Tag, low endotoxin |  |
|

|

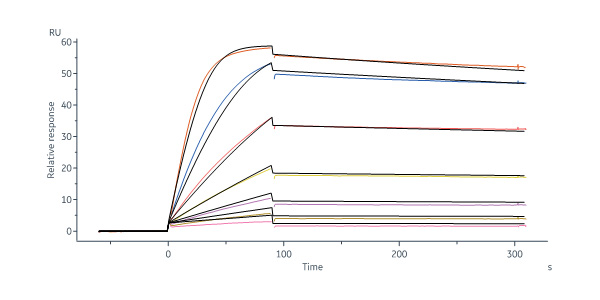
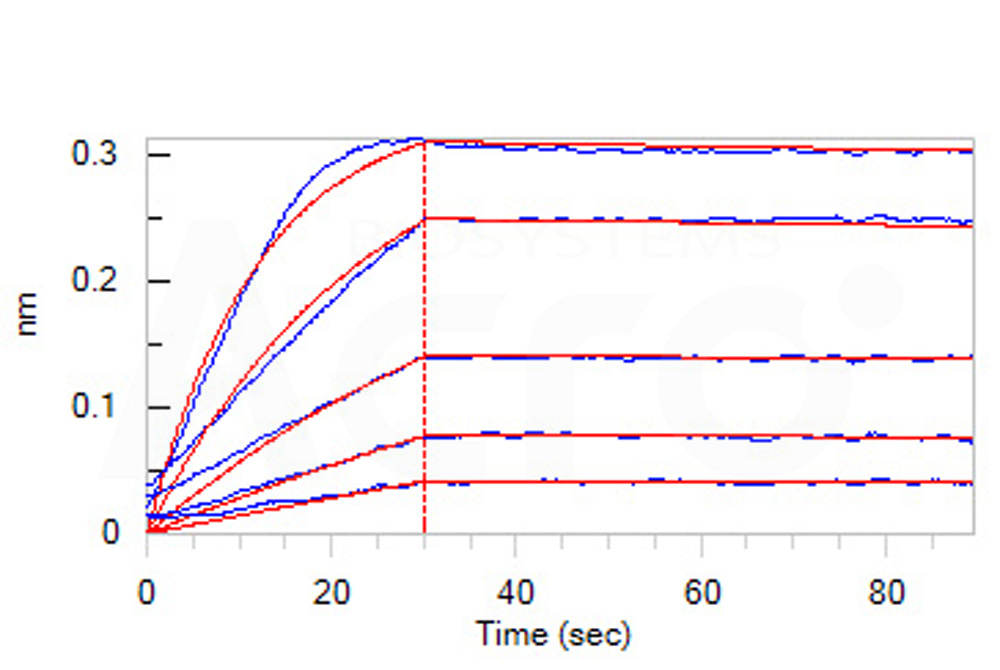
Human VEGF R1 Protein, Fc Tag (Cat. No. VE1-H5253) captured on Protein A Chip can bind Human VEGF-B, His Tag (Cat. No. VE6-H5225) with an affinity constant of 0.52 nM as determined in a SPR assay (Biacore 8K) (Routinely tested).
| Name | Research Code | Research Phase | Company | First Brand Name | First Approved Country | First Indication | First Approved Company | First Approved Date | Indications | Clinical Trials |
|---|---|---|---|---|---|---|---|---|---|---|
| Plitidepsin | APLD | Approved | Pharma Mar Sa | Aplidin | Australia | Multiple Myeloma | null | 2018-12-11 | Leukemia; Solid tumours; Coronavirus Disease 2019 (COVID-19); Multiple Myeloma; Liposarcoma; Prostatic Neoplasms; Primary Myelofibrosis; Lymphoma | Details |
| Nintedanib Esylate | BIBF-1120 | Approved | C.H. Boehringer Sohn Ag & Co. Kg | Ofev, Vargatef | EU | systemic sclerosis-associated interstitial lung disease | Boehringer Ingelheim International Gmbh | 2014-10-15 | Gliosarcoma; Breast Neoplasms; Sarcoma; systemic sclerosis-associated interstitial lung disease; Adenocarcinoma, Clear Cell; Peritoneal Neoplasms; Colorectal Neoplasms; Astrocytoma; Hepatic Insufficiency; Genital Neoplasms, Female; Silicosis; Prostatic Neoplasms; Carcinoma, Squamous Cell; Appendiceal Neoplasms; Fallopian Tube Neoplasms; Leukemia, Myeloid, Acute; Uterine Neoplasms; Endometrial Neoplasms; Carcinoma, Non-Small-Cell Lung; Adenocarcinoma; Carcinoma, Endometrioid; Carcinoma, Hepatocellular; Colonic Neoplasms; Ovarian Neoplasms; Telangiectasia, Hereditary Hemorrhagic; Esophageal Neoplasms; Rejection of lung transplantation; Carcinoid Tumor; Endometrial Stromal Tumors; Carcinoma, Renal Cell; Radiation Pneumonitis; Neoplasms; Idiopathic Pulmonary Fibrosis; Solid tumours; Scleroderma, Systemic; Glioblastoma; Small Cell Lung Carcinoma; Mesothelioma; Neuroendocrine Tumors; Pulmonary Fibrosis; Lung Diseases, Interstitial; Multiple Myeloma; Asbestosis; Oligodendroglioma | Details |
| Lenvatinib Mesylate | ER-203492-00; E-7080; MK-7902 | Approved | Eisai Co Ltd | Kisplyx, Lenvima, Lenvima/Kisplyx, 乐卫玛 | Japan | Carcinoma, Renal Cell | Merck Sharp & Dohme Corp | 2015-02-13 | Liver Diseases; Paraganglioma; Neoplasm Metastasis; Thyroid Cancer, Papillary; Melanoma; Carcinoma, Hepatocellular; Carcinoma, Non-Small-Cell Lung; Glioma; Lymphoma; Endometrial Neoplasms; Thyroid Neoplasms; Hepatic Insufficiency; Osteosarcoma; Cholangiocarcinoma; Breast Neoplasms; Solid tumours; Neuroendocrine Tumors; Carcinoma, Adenoid Cystic; Adenocarcinoma, Follicular; Kidney Diseases; Neoplasms; Adenocarcinoma of Lung; Thyroid Carcinoma, Anaplastic; Pheochromocytoma; Renal Insufficiency; Esophageal Neoplasms; Carcinoma, Renal Cell; Ovarian Neoplasms; Liver Neoplasms; Biliary Tract Neoplasms | Details |
| Regorafenib | DAST; BAY-73-4506 | Approved | Bayer AG | Stivarga, Resihance | Mainland China | Gastrointestinal Stromal Tumors | Bayer Pharma Ag | 2012-09-27 | Leukemia, Myeloid, Acute; Sarcoma; Gastrinoma; Osteosarcoma; Sarcoma, Ewing; Colorectal Neoplasms; Peritoneal Neoplasms; Gastrointestinal Stromal Tumors; Bile Duct Neoplasms; Fallopian Tube Neoplasms; Esophageal adenocarcinoma; Adenoma; Thyroid Neoplasms; Lung Neoplasms; Thymoma; Carcinoma, Hepatocellular; Adenocarcinoma; Melanoma; Neoplasm Metastasis; Gastrointestinal Neoplasms; Somatostatinoma; Carcinoma, Islet Cell; Ovarian Neoplasms; Liver Neoplasms; Hemangiosarcoma; Esophageal Neoplasms; Stomach Neoplasms; Carcinoma, Renal Cell; Rectal Neoplasms; Carcinoid Tumor; Insulinoma; Solid tumours; Colonic Neoplasms; Neoplasms; Carcinoma, Ovarian Epithelial; Pancreatic Neoplasms; Small Cell Lung Carcinoma; Glioblastoma; Carcinoma, Transitional Cell; Carcinoma, Adenoid Cystic; Glucagonoma | Details |
| Anlotinib Dihydrochloride | AL-3818 | Approved | Advenchen Laboratories Llc, Jiangsu Chia Tai-Tianqing Pharmaceutical Co Ltd | 福可维 | Mainland China | Thyroid Neoplasms | Jiangsu Chia Tai-Tianqing Pharmaceutical Co Ltd | 2018-05-08 | Esophageal Squamous Cell Carcinoma; Bone Neoplasms; Peritoneal Neoplasms; Colorectal Neoplasms; Hepatic Insufficiency; Gastrointestinal Stromal Tumors; Urologic Neoplasms; Sarcoma, Alveolar Soft Part; Endometrial Neoplasms; Gallbladder Neoplasms; Bile Duct Diseases; Thyroid Neoplasms; Fallopian Tube Neoplasms; Lung Neoplasms; Glioma; Carcinoma, Non-Small-Cell Lung; Uterine Cervical Neoplasms; Melanoma; Carcinoma, Hepatocellular; Osteoma; Small Cell Lung Carcinoma; Head and Neck Neoplasms; Solid tumours; Biliary Tract Neoplasms; Drug-Related Side Effects and Adverse Reactions; Ovarian Neoplasms; Esophageal Neoplasms; Thoracic Neoplasms; Carcinoma, Renal Cell; Stomach Neoplasms; Leiomyosarcoma; Lymphoma, Large B-Cell, Diffuse; Neoplasms; Carcinoma, Ovarian Epithelial; Sarcoma, Synovial; Neuroendocrine Tumors; Liver Diseases; Sarcoma; Medullary thyroid cancer (MTC); Nasopharyngeal Carcinoma | Details |
| Sorafenib Tosylate | NSC-724772; BAY-43-0006; BAY-43-9006; BAY-54-9085 | Approved | Onyx Pharmaceuticals Inc | Nexavar, 多吉美 | EU | Thyroid Neoplasms | Bayer AG | 2005-12-01 | Liver Neoplasms; Leukemia, Myeloid; Lymphoma, B-Cell, Marginal Zone; Rhabdomyosarcoma; Head and Neck Neoplasms; Leukemia, Myelogenous, Chronic, BCR-ABL Positive; Fibromatosis, Aggressive; Leiomyosarcoma; Kidney Neoplasms; Leukemia, Erythroblastic, Acute; Solid tumours; Lymphoma, T-Cell, Peripheral; Ovarian Neoplasms; Recurrence; Carcinoma, Renal Cell; Esophageal Neoplasms; Vipoma; Rectal Neoplasms; Squamous Cell Carcinoma of Head and Neck; Histiocytoma, Malignant Fibrous; Carcinoma, Islet Cell; Hemangiosarcoma; Carcinoid Tumor; Carcinoma; Insulinoma; Stomach Neoplasms; Neoplasms; Pancreatic Neoplasms; Multiple Endocrine Neoplasia Type 2a; Hypertension, Portal; Carcinoma, Transitional Cell; Carcinoma, Verrucous; Thyroid Carcinoma, Anaplastic; Myelodysplastic Syndromes; Glioblastoma; Carcinoma, Ovarian Epithelial; Leukemia, Myelomonocytic, Acute; Colonic Neoplasms; Lymphoma, Large B-Cell, Diffuse; Lymphomatoid Granulomatosis; Wilms Tumor; Leukemia, Myelomonocytic, Chronic; Lymphoma, Large-Cell, Immunoblastic; S | Details |
| Axitinib | AG-013736; CLS-AX; AG-13736; PF-01367866 | Approved | Pfizer Pharmaceuticals Ltd (China) | Inlyta, 英立达 | Mainland China | Carcinoma, Renal Cell | Pfizer Europe Ma Eeig | 2012-01-27 | Lymphoma; Carcinoma, Adenoid Cystic; Breast Neoplasms; Cholangiocarcinoma; Prostatic Neoplasms; Sarcoma; Colorectal Neoplasms; Hepatic Insufficiency; Carcinoma, Pancreatic Ductal; Thyroid Neoplasms; Carcinoma, Ductal; Leukemia, Myeloid, Acute; Lung Neoplasms; Carcinoma, Hepatocellular; Paraganglioma; Carcinoma, Non-Small-Cell Lung; Melanoma; Adenocarcinoma; Macular Degeneration; Adrenal Cortex Neoplasms; Kidney Neoplasms; Carcinoid Tumor; Carcinoma, Renal Cell; Pheochromocytoma; Stomach Neoplasms; Carcinoma; Squamous Cell Carcinoma of Head and Neck; Neoplasms; Solid tumours; Glioblastoma; Pancreatic Neoplasms; Skin Neoplasms; Myelodysplastic Syndromes; Nasopharyngeal Neoplasms; Prostatic Neoplasms, Castration-Resistant; Neuroendocrine Tumors; Mesothelioma | Details |
| Fruquintinib | HMPL-013 | Approved | Hutchison Medipharma Ltd | 爱优特, Elunate | Macau | Colorectal Neoplasms | Hutchison Medipharma Ltd | 2018-09-04 | Kidney Diseases; Carcinoma, Non-Small-Cell Lung; Lung Neoplasms; Colorectal Neoplasms; Hepatic Insufficiency; Breast Neoplasms; Sarcoma; Triple Negative Breast Neoplasms; Pancreatic Neoplasms; Solid tumours; Colonic Neoplasms; Neoplasms; Rectal Neoplasms; Carcinoma, Renal Cell; Esophageal Neoplasms; Stomach Neoplasms; Biliary Tract Neoplasms | Details |
| Cabozantinib S-malate | XL-184; BMS-907351 | Approved | Exelixis Inc | Cometriq, Cabometyx | Japan | Carcinoma, Renal Cell | Takeda | 2012-11-29 | Osteosarcoma; Thyroid Neoplasms; Gliosarcoma; Colorectal Neoplasms; Peritoneal Neoplasms; Bile Duct Neoplasms; Hepatic Insufficiency; Astrocytoma; Breast Neoplasms; Sarcoma, Ewing; Lymphoma; Carcinoma, Adenosquamous; Adenocarcinoma, Clear Cell; Sarcoma, Clear Cell; Medullary thyroid cancer (MTC); Cholangiocarcinoma; Prostatic Neoplasms; Neurofibroma, Plexiform; Urethral Neoplasms; Carcinoma, Neuroendocrine; Meningioma; Carcinoma, Non-Small-Cell Lung; Paraganglioma; Melanoma; Carcinoma, Endometrioid; Thyroid Cancer, Papillary; Adenocarcinoma; Neoplasms, Germ Cell and Embryonal; Carcinoma, Hepatocellular; Neuroblastoma; Glioma; Leukemia, Myeloid, Acute; Carcinoma, Squamous Cell; Endometrial Neoplasms; Lung Neoplasms; Sarcoma, Alveolar Soft Part; Uterine Neoplasms; Fallopian Tube Neoplasms; Carcinoid Tumor; Neurofibromatoses; Seminoma; Carcinoma, Transitional Cell; Glioblastoma; Rejection of liver transplantation; Carcinoma, Merkel Cell; Hepatoblastoma; Pain; Pheochromocytoma; Neoplasms; Carcinoma, Renal Cell; B | Details |
| Pazopanib Hydrochloride | GSK-786034; GW-786034B; SB-786034; GW-786034 | Approved | Glaxosmithkline Plc, Novartis Pharma Ag | 维全特, Armala, Votrient, Patorma | Mainland China | Carcinoma, Renal Cell | Novartis Pharma Schweiz Ag | 2009-10-19 | Peritoneal Neoplasms; Lymphoma; Uterine Neoplasms; Lung Neoplasms; Carcinoma, Small Cell; Thyroid Neoplasms; Gastrointestinal Stromal Tumors; Gliosarcoma; Carcinoma, Mucoepidermoid; Genital Neoplasms, Female; Uterine Cervical Diseases; Choriocarcinoma; Colorectal Neoplasms; Neuroblastoma; Osteosarcoma; Gastrinoma; Psoriasis; Brain Neoplasms; Urethral Neoplasms; Breast Neoplasms; Medullary thyroid cancer (MTC); von Hippel-Lindau Disease; Gastrointestinal Neoplasms; Carcinoma, Hepatocellular; Melanoma; Endodermal Sinus Tumor; Neoplasms, Germ Cell and Embryonal; Paraganglioma; Thyroid Cancer, Papillary; Neoplasm Metastasis; Uterine Cervical Neoplasms; Breast Neoplasms, Male; Prostatic Neoplasms; Macular Degeneration; Carcinoma, Non-Small-Cell Lung; Fallopian Tube Neoplasms; Leukemia, Myeloid, Acute; Glioma; Germinoma; Carcinoma, Neuroendocrine; Carcinoma, Embryonal; Carcinoma, Squamous Cell; Telangiectasia, Hereditary Hemorrhagic; Thyroid Carcinoma, Anaplastic; Neoplasms; Carcinoid Tumor; Herpes Genitalis; Pheoc | Details |
| Sunitinib Malate | PHA-290940; SU-010398; SU-11248; PNU-290940AD; PHA-290940AD; GB-102; PNU-290940; SU-011248-L-malate salt | Approved | Pfizer Pharmaceuticals Ltd (China) | 索坦, Sutent | Japan | Pancreatic neuroendocrine tumors (pNET) | Pfizer Inc | 2006-01-26 | Teratoma; Ovarian Neoplasms; Fibromatosis, Aggressive; Solid tumours; Intestinal Neoplasms; Kidney Neoplasms; Liver Neoplasms; Leiomyosarcoma; Fibrosarcoma; HIV Infections; Head and Neck Neoplasms; Lymphoma, B-Cell, Marginal Zone; Lymphoma, B-Cell; Leukemia, Myeloid, Accelerated Phase; Leukemia; Leukemia, Myelogenous, Chronic, BCR-ABL Positive; Lymphoma, T-Cell, Peripheral; Ependymoma; Carcinoma, Renal Cell; Histiocytoma, Malignant Fibrous; Carcinoma; Hemangioblastoma; Carcinoma, Islet Cell; Stomach Neoplasms; Squamous Cell Carcinoma of Head and Neck; Thoracic Neoplasms; Leukemia, Hairy Cell; Abdominal Neoplasms; Esophageal Neoplasms; Polycythemia Vera; Pelvic Neoplasms; Pheochromocytoma; Glioblastoma; Neurofibromatoses; Pancreatic Neoplasms; Pancreatic neuroendocrine tumors (pNET); Salivary Gland Neoplasms; Small Cell Lung Carcinoma; Leukemia, Myelomonocytic, Chronic; Myelodysplastic Syndromes; Hodgkin Disease; Lymphoma, Large B-Cell, Diffuse; Carcinoma, Ovarian Epithelial; Carcinoma, Papillary; Neoplasms; I | Details |
| Tivozanib | AV-951; KRN-951; ASP-4130; Kil-8951 | Approved | Kyowa Hakko Kirin Co Ltd | Fotivda | United States | Carcinoma, Renal Cell | Aveo Pharmaceuticals Inc | 2017-08-24 | Cholangiocarcinoma; Gastrointestinal Neoplasms; Carcinoma, Hepatocellular; Carcinoma, Non-Small-Cell Lung; Metastatic breast cancer; Fallopian Tube Neoplasms; Bile Duct Neoplasms; Peritoneal Neoplasms; Hepatic Insufficiency; Colorectal Neoplasms; Liver Neoplasms; Sarcoma; Breast Neoplasms; Carcinoma, Ovarian Epithelial; Triple Negative Breast Neoplasms; Carcinoma, Renal Cell; Biliary Tract Neoplasms; Solid tumours; Ovarian Neoplasms | Details |
| Name | Research Code | Research Phase | Company | Indications | Clinical Trials |
|---|---|---|---|---|---|
| VEGFR-1/2 peptide vaccine (Keio University) | Phase 2 Clinical | Keio University | Neurofibromatoses; Brain Neoplasms; Glioma | Details | |
| Sorafenib Tosylate/MG-K10 | MG-D-1609 | Phase 2 Clinical | Solid tumours | Details | |
| Ilorasertib | ABT-348; A-9686600; Abbott-968660 | Abbvie Inc | Details | ||
| R-1530 | RG1530; R-1530; RG-1530 | F. Hoffmann-La Roche Ltd | Details | ||
| ODM-203 | ODM-203 | Orion Corp | Details | ||
| OCV-101 | OCV-101; OTS-11101 | Phase 2 Clinical | Oncotherapy Science, Inc | Pancreatic Neoplasms | Details |
| Lucitanib | AL-3810; CO-3810; S-80881 | Phase 3 Clinical | Advenchen Laboratories Nanjing Ltd | Solid tumours; Stomach Neoplasms; Small Cell Lung Carcinoma; Breast Neoplasms; Nasopharyngeal Carcinoma; Colorectal Neoplasms; Genital Neoplasms, Female; Thymus Neoplasms; Carcinoma, Small Cell; Lung Neoplasms; Carcinoma, Non-Small-Cell Lung; Carcinoma, Hepatocellular | Details |
| Glesatinib | 7Q29OXD98N; MG-90265H9; MG-90265gly; MG-90265; MG-90265X; MGCD-265 | Phase 2 Clinical | Mirati Therapeutics Inc | Neoplasms; Carcinoma, Non-Small-Cell Lung | Details |
| Cediranib | AZD2171; AZD-2171; NSC-732208 | Phase 3 Clinical | Astrazeneca Plc | Ovarian Neoplasms; Solid tumours; Ependymoma; Biliary Tract Neoplasms; Liver Neoplasms; Head and Neck Neoplasms; Leukemia; Leiomyosarcoma; Solitary Fibrous Tumor, Pleural; Medulloblastoma; Lymphoma, B-Cell; Lymphoma, B-Cell, Marginal Zone; Lymphoma, T-Cell, Peripheral; Cystadenocarcinoma, Serous; Carcinoma, Renal Cell; Rectal Neoplasms; Carcinoma; Stomach Neoplasms; Rhabdoid Tumor; Spinal Cord Neoplasms; Cystadenocarcinoma, Mucinous; Squamous Cell Carcinoma of Head and Neck; Leukemia, Hairy Cell; Cystadenoma, Serous; Glioblastoma; Triple Negative Breast Neoplasms; Colonic Neoplasms; Pancreatic Neoplasms; Small Cell Lung Carcinoma; Neoplasms; Hodgkin Disease; Hypopharyngeal Neoplasms; Leukemia-Lymphoma, Adult T-Cell; Carcinoma, Transitional Cell; Carcinoma, Ovarian Epithelial; Myelodysplastic Syndromes; Lymphoma, Large B-Cell, Diffuse; Nasopharyngeal Neoplasms; Salivary Gland Neoplasms; Carcinoma, Verrucous; Lymphomatoid Granulomatosis; Lymphoma, Large-Cell, Immunoblastic; Oligodendroglioma; Ascites; Uveal mel | Details |
| Dual-targeting VEGFR1 and PD-L1 CAR-T cells Therapy (Sichuan University) | Phase 1 Clinical | Sichuan University | Serositis; Ascites | Details | |
| Famitinib Malate | SHR-1020 | Phase 3 Clinical | Jiangsu Hengrui Medicine Co Ltd | Cholangiocarcinoma; Neoplasm Metastasis; Gastrointestinal Neoplasms; Adenocarcinoma; Uterine Cervical Neoplasms; Carcinoma, Non-Small-Cell Lung; Thyroid Neoplasms; Endometrial Neoplasms; Lung Neoplasms; Urologic Neoplasms; Genital Neoplasms, Female; Gastrointestinal Stromal Tumors; Colorectal Neoplasms; Ovarian Neoplasms; Nasopharyngeal Carcinoma; Breast Neoplasms; Triple Negative Breast Neoplasms; Carcinoma, Transitional Cell; Pancreatic neuroendocrine tumors (pNET); Nasopharyngeal Neoplasms; Idiopathic Pulmonary Fibrosis; Neoplasms; Carcinoma, Renal Cell; Solid tumours; Biliary Tract Neoplasms | Details |
| Sitravatinib | IND-155305; MG-516; MG-91516; MGCD-516 | Phase 3 Clinical | Mirati Therapeutics Inc | Breast Neoplasms; Carcinoma, Hepatocellular; Gastrointestinal Neoplasms; Uterine Cervical Neoplasms; Carcinoma, Non-Small-Cell Lung; Lung Neoplasms; Carcinoma, Squamous Cell; Mouth Neoplasms; Endometrial Neoplasms; Esophageal Squamous Cell Carcinoma; Hepatic Insufficiency; Ureteral Neoplasms; Kidney Neoplasms; Liposarcoma; Lung Diseases; Uveal melanoma; Triple Negative Breast Neoplasms; Carcinoma, Transitional Cell; Neoplasms; Carcinoma; Squamous Cell Carcinoma of Head and Neck; Carcinoma, Renal Cell; Biliary Tract Neoplasms; Solid tumours | Details |
This web search service is supported by Google Inc.
